What do you know about QC, IQC, IPQC, QA in the factory, do you know?
First, QC and QA
QC: Quality Control, quality control, product quality inspection, general analysis of the analysis, improvement and non-conforming product control personnel after the quality problem.
Generally includes:
IQC (Incoming Quality Control) means the quality control of incoming materials, referred to as incoming material control.
IPQC (In Put Process Quality Control) means process control, which refers to the quality control of the final packaging process of products from the production of materials into production channels.
FQC (Finish or Final Quality Control) finished product quality inspection
OQC (Out Quality control) finished product inspection
DQC (Design Quality Control) design quality control
MQC (Manufacture Quality Control) process inspection

QA: Quality Assurance, Quality Assurance, ensuring product quality without problems by establishing and maintaining a quality management system.
Generally includes:
IDQA (Design Quality Assurance) design quality assurance, such as DQA manager (design quality certification manager)
QE (Quality Engineer) Quality Engineer
JQE (Joint Quality Engineer) client quality engineer, the quality engineer who is employed by the supplier to work for the customer, is the eyes and ears of the customer SQE.
SQE (Supplier Quality Engineer) supplier quality engineer.
Second, the difference between QA and QC
QA not only needs to know where the problem lies, but also how to solve these problem solutions and how to prevent it in the future; QC needs to know that it is only controlled if there is a problem, but it is not necessary to know why it is controlled.
[ Inappropriate analogy ] QC is the police, QA is the judge, QC can only take the violation of the law, it can not prevent others from committing crimes and finally convicting others, and the judge is to enact laws to prevent crime, according to the law. Dispute the results of the disposition.
Summarize it:
QC is mainly based on quality inspection activities after the event. The default error is allowed, and it is expected to find and select errors. QA is mainly a prior quality assurance activity, mainly based on prevention, and it is expected to reduce the probability of occurrence of errors.
QC is the operation technology and activities adopted to meet the quality requirements of the product. It includes inspection, correction and feedback. For example, QC will detect the defective products and remove them, and then feedback the bad information to relevant departments for improvement measures. Therefore, the control scope of QC is mainly inside the factory, and its purpose is to prevent the non-conforming products from being put into, reordered, and shipped out, to ensure that the products meet the quality requirements and only qualified products can be delivered to the customers.
QA is to provide trust to meet customer requirements. Even if the customer is convinced that the product you provide can meet his requirements, it is necessary to review customer requirements, product development, order and material procurement, incoming inspection, and production process from the beginning of market research and later. Control and shipment, after-sales service and other stages leave evidence to confirm that every step of the factory activities are carried out according to customer requirements.
The purpose of QA is not to ensure product quality, but to ensure product quality is the task of QC.
QA is mainly to provide conviction, so it is necessary to manage the whole process of understanding customer requirements from start to after-sales service. This requires enterprises to establish a quality control system, formulate corresponding documents to regulate the activities of each process and leave evidence of activity implementation to provide trust.
This kind of trust can be divided into two kinds : internal and external : even if the customer is assured, it is believed that the factory produces and delivers the product according to its requirements; the internal is to reassure the factory owner, because the boss is the first responsible person of product quality, and the product has a quality accident. He has to bear full responsibility. This is also the main requirement for countries to formulate product quality laws, so that enterprises can really pay attention to quality. Therefore, in order to avoid bearing the quality responsibility, the boss must document the activities and leave evidence. However, the internal staff of the factory is not required to operate according to the documents. The boss cannot understand them one by one. This requires QA to replace him for auditing to understand whether the documents are complied with, so that the boss can believe that the activities of the factory are carried out according to the documents. Reassure him.
Therefore, the main difference between QC and QA is that the former guarantees product quality and the latter, and the latter establishes the system and ensures that the system operates as required to provide internal and external trust.
At the same time, QC and QA have the same point: QC and QA must be verified. For example, QC tests the product according to the standard to verify whether the product meets the requirements. QA conducts internal audit to verify whether the system meets the standard requirements, and QA Shipment audit and reliability testing is to verify whether the product has been carried out in accordance with the regulations, whether it can meet the requirements, to ensure that the products delivered by the factory are qualified and in compliance with relevant regulations.
The most important responsibility of QC is to monitor the finished products (mainly: Raw material, in-process goods, finish goods, In-process audit), focusing on Detect defect through Sample Inspection.
Third, IPQC and IQC
QC has the distinction between IPQC and IQC.
IPQC :IN PROCESS QUALITY CONTROL Process Quality Control
IQC: IN COME QUALITY CONTROL Feed Quality Control
Its responsibilities are as follows:
IPQC responsibilities:
1. Inspect the products in the production process and make a record
2. Fill in the inspection report according to the inspection record
3. Suggest improvements to the problems found in the inspection
IQC responsibilities:
1. Inspect raw materials strictly according to inspection standards
2. Fill in the inspection record form truthfully
3. Inspection equipment maintenance and maintenance
4. Reporting of abnormal raw materials
5. Identification of raw materials
6. Responsible for the receipt of the inspection report of the warehouse material
7. Responsible for re-inspecting the inventory materials of the warehouse for material quality problems in the production line complaints
QA is quality supervision / monitoring
1. Responsible for the overall work of the department, organize the implementation of GMP quality management regulations, and timely submit product quality opinions and suggestions for improvement to business leaders.
2. Ensure that the products of the company are produced in compliance with GMP requirements.
3. Responsible for the implementation, correction and prevention of people and things related to quality throughout the enterprise.
4. The instructions for the production configuration are reviewed and approved after the designated personnel of the department have signed the audit.
5. Review and approve the test results.
6. Review the pilot program and conclusions of new product development and process improvement.
7. Review the relevant technical and quality written materials submitted to the drug regulatory authority.
8. Review the batch records and conclude whether the finished product is shipped.
9. Responsible for organizing the development of quality standards and other documents for raw materials and packaging materials.
10. Review the non-conforming product handling procedures.
11. Due to the needs of quality management, we will organize the preparation of new technical standards or discuss the revision of technical standards with relevant departments.
12. Review the production process specifications, batch production records, batch packaging records of each product, and determine the finished product distribution.
13. Deal with product quality issues with user complaints, assign personnel or return to the user in person. The meeting will be held internally, and the relevant departments will study and improve the quality issues, and report the complaints and the results of the complaints to the person in charge of the company.
14. Regularly (at least once a year) will conduct a comprehensive GMP inspection with the General Works Office and the Production Department, and report the inspection to the person in charge of the enterprise in a timely manner.
phenylthiophosphonic dichloride Basic Information
Product Name: Phenylthiophosphonic Dichloride
CAS: 3497-00-5
MF: C6H5Cl2PS
MW: 211.05
Mol File: 3497-00-5.mol
Phenylthiophosphonic Dichloride Structure
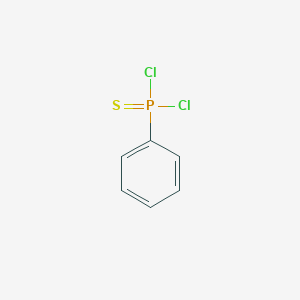
Melting point 76-77 °C
Boiling point 270 °C
density 1,4 g/cm3
refractive index 1.6178-1.6182
Phenylthiophosphonic Dichloride CAS No.3497-00-5
phenylthio phosphonic dichloride,phenylthiophosphonic dichloride,Dichlorophenylphosphine sulfide
ShanDong YingLang Chemical Co.,LTD , https://www.sdylhgtrade.com
