The role and mechanism of pre-oxidation and permeation before ion nitriding
42CrMo steel has good comprehensive properties, such as high strength and toughness, good hardenability, etc. It has good toughness and fatigue resistance after quenching and is widely used in gears. In engineering applications, in order to meet the design requirements of gear surface wear resistance and good toughness of the heart, surface heat treatment is generally required to improve its service performance. Ion nitriding is a widely used chemical heat treatment technique. However, the shortcoming is that in practical applications, the ion nitriding cycle tends to be long, and some even reach dozens of hours or more. It has caused great energy consumption and reduced production efficiency. Pre-oxidation and percolation have been applied in gas nitriding and have achieved good infiltration effect. The so-called pre-oxidation (PO) is the formation of a thin layer of dense oxide film on the surface of the workpiece by air oxidation. In the gas nitriding stage, on the one hand, under the action of a reducing substance, the oxide is continuously reduced and combined with a reactive nitrogen atom to form a nitride, which is concentrated on the surface. On the other hand, since the oxide is continuously reduced to form a loose porous structure, the diffusion channel of the nitrogen atom is expanded, which increases the chance of adsorbing nitrogen atoms on the surface. Under this action, the oxide is converted to a nitride, increasing the thickness of the effective nitride layer. Studies have shown that the oxide layer not only does not hinder nitriding as expected, but has a significant infiltration effect. Since pre-oxidation in an ion nitriding furnace is accompanied by surface sputtering, it is worthwhile to study whether it can act as a seepage in gas nitriding.
In this paper, the pre-oxidation technology was applied to the ion nitriding of 42CrMo steel. The influence of pre-oxidation process on ion nitriding was studied. It was found that pre-oxidation has the effect of infiltration on ion nitriding. Combined with the observation of surface oxide morphology and surface free energy after pre-oxidation, the mechanism of pre-oxidation on ion nitriding was discussed.
Experimental materials and methods
The test material is a temperate 42CrMo steel, and its chemical composition (mass fraction, %) is: 0.30-0. 43 C; 0. 15-0. 35 Si; 0. 15-0. 25 Mo; -1. 0Mn; 0. 8-1. 01 Cr; the balance is Fe. The hardness of the substrate is 350 HV0. 05. Specimens with a size of 10 mm × 10 mm × 10 mm were cut by wire cutting for tissue observation and hardness testing. The surface of the sample is first smoothed with sandpaper, polished with Cr 2 O 3 polishing powder to the mirror surface, and finally washed with ultrasonic ethanol in ultrasonic wave, and used.
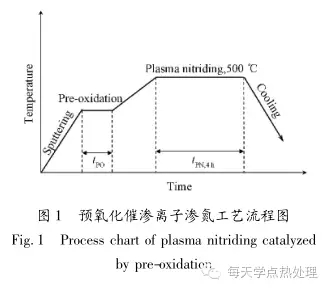
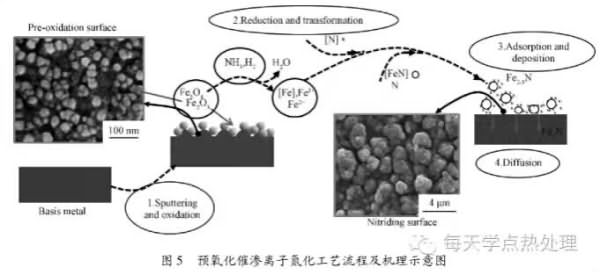
The process of pre-oxidation and nitriding ion nitriding is mainly divided into the following three steps (see Figure 1): firstly, hydrogen is introduced into the surface of the sample, and the surface of the sample is sputter-heated and cleaned; when the set temperature is reached, air is introduced for pre-oxidation. The air flow rate is 3 L/min; after the pre-oxidation is completed, nitrogen and hydrogen are introduced at a ratio of 1:3 to enter the ion nitriding stage, and then cooled with the furnace. The preoxidation process parameters used are shown in Table 1.
The microstructure and thickness of the nitrided layer were observed by DMI-3000M optical microscope. The phase composition of the layer was tested by D/max-2500 X-ray diffractometer. The surface morphology of the surface was observed by SUPRA55 field emission electron microscopy. The contact angle measurement was performed with JC2000D1 type. The instrument tests the pre-oxidized surface contact angle. The surface contact angle test method is a droplet method: the droplet is dropped on the surface of the sample through a syringe needle, and the contact angle is measured by a tangent method, and each sample is measured at five different positions, and the average value is taken as the test piece. Contact angle. Using HXD-1000TMC micro hardness tester
Results and discussion
2. 1 Analysis of microstructure and thickness of nitrided layer
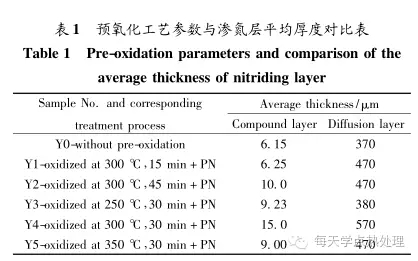
Figure 2 shows the cross-section microstructure of the sample with the same process ion nitriding without pre-oxidation and after different pre-oxidation processes. It can be seen that the thickness of the compound layer is only 6.15 μm when the pre-oxidation is carried out under the same nitriding process. After the pre-oxidation treatment, the compound layers have different degrees of increase. When the pre-oxidation process is at 300 °C for 30 min, the thickness of the compound layer reaches a maximum of 15 μm, which is more than twice that of conventional ion nitriding (without pre-oxidation). At the same time, the cross-section microhardness test analysis results (see Table 1 for details) show that under the same nitriding process conditions, the effective diffusion layer thickness is 370 μm without pre-oxidation. After the pre-oxidation treatment, the thickness of the effective diffusion layer is increased. When the pre-oxidation process is at 300 °C for 30 min, the thickness of the effective diffusion layer reaches a maximum of 570 μm, which is significantly higher than the thickness of the effective diffusion layer of conventional ion nitriding (without pre-oxidation).
2. 2 XRD phase analysis
Figure 3 shows the surface X-ray diffraction pattern of 42CrMo steel under four treatment conditions. It can be seen from PO that after pre-oxidation at 300 °C for 30 min, only a thin oxide is formed on the surface, and the matrix α-Fe phase is still the main phase. It can be seen from Y4 that the PO process is pre-oxidized and then subjected to ion nitridation, and the surface phase of the sample is completely converted into a nitride phase, including a Fe 4 N phase and a Fe 2 -3 N phase. However, it can be seen from Y2 and Y5 that when the pre-oxidation conditions are 300 °C, 45 min or 350 °C, 30 min, the surface of the sample contains not only nitride but also a small amount of oxide while ion nitriding. α-Fe phase. The main reason for the difference in surface phase after different process pre-oxidation + the same process ion nitriding may be: ion nitriding stage is the process in which the oxide is reduced and decomposed, and finally replaced by nitride, due to different processes pre-oxidation treatment The thickness and microstructure of the oxide formed on the surface are different, causing a difference.

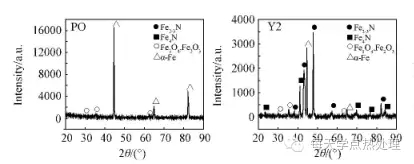
Fig. 3 X-ray diffraction spectrum of 42CrMo steel treated by different process conditions
(PO) pre-oxidation at 300 °C for 30 min without nitriding treatment; (Y2) oxidation at 300 °C for 45 min + PN;
(Y5) Oxidation at 350 °C for 30 min + PN (Y4) Oxidation at 300 °C for 30 min + PN
The oxides are incompletely reduced and decomposed during the nitriding stage and therefore cannot be completely replaced by nitrides. The α-Fe phase appearing under the pre-oxidation conditions of Y2 and Y5 may be an iron matrix which has not been formed by the reduction of Fe from the oxide or has been detected due to insufficient thickness of the nitride layer, that is, the oxide is reduced and decomposed. And in the dynamic process of being replaced by nitride.
This further demonstrates the existence of a separate process for the reduction of oxides by hydrogen atoms into Fe during nitriding, thus providing another way to combine reactive nitrogen with Fe. 2. 3 Pre-oxidation surface morphology analysis Figure 4 shows the surface morphology of 42CrMo steel after pre-oxidation and non-pre-oxidation. Compared with the surface morphology without pre-oxidation after hydrogen sputtering as shown in Fig. 4(a), oxides of different morphologies are formed on the surface of the sample after pre-oxidation. Under the conditions shown in Fig. 4(b), the nano-scale spherical oxide particles are more evenly distributed on the surface of the substrate, and the surface exhibits a large number of micro-cracks and holes. On the one hand, the nano-spherical oxide particles have a large specific surface area and interfacial energy, which is favorable for the adsorption and crystallization of the active nitrogen atoms. On the other hand, the matrix generates a large number of defects such as cracks and holes, which can adhere to a large amount of active nitrogen atoms and provide a channel for the diffusion of nitrogen atoms. As the pre-oxidation time increases or the temperature increases, the oxide particles grow and combine with each other. One piece forms a dense oxide film as shown in Figures 4(c) and 4(d). As can be seen in Fig. 4(d), the oxide is distributed in a lamellar shape, and a string of oxides is formed at the boundary of the sheet and the sheet to have a higher density. The high-density oxide film needs to be impeded by micro-cracks and the like under the subsequent ion-nitridation reduction and sputtering. In this process, the oxide is stripped, the oxidative decomposition is not complete, and the oxide is finally formed. Coexisting with the nitride on the surface, the coexistence of the oxide phase and the nitride phase shown by Y2 and Y5 in Fig. 3 proves that the microstructure oxide is not easily completely replaced by the nitride.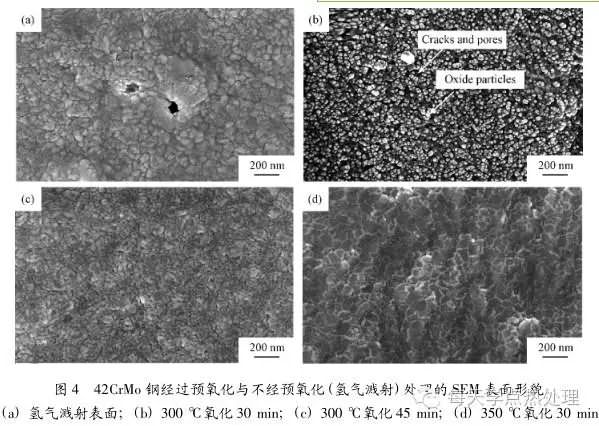
2. 4 Calculation of free energy of pre-oxidized surface
Surface free energy is an important physical quantity that characterizes surface activity and has an important influence on the surface adsorption of the sample. By measuring the contact angle, the surface free energy after different pre-oxidation processes was calculated using the Owens-Wendt formula. The surface free energy calculation formula is as follows

The γ f is the surface free energy of the formamide, γ df and γ pf are the dispersion component and the polar component of the surface energy, respectively, and the dispersion component value is 39.5 mJ/m 2 , and the polar component value is 18. 7 mJ. /m 2. Γw is the surface free energy of deionized water, γ dw and γ pw are the dispersion component and the polar component of the surface energy, respectively, the dispersion component value is 21. 8 mJ/m 2 , and the polar component value is 51.0 mJ/ m 2 [12]. Θw and θ f are the contact angles of deionized water and formamide, respectively. The test results are shown in Table 2.
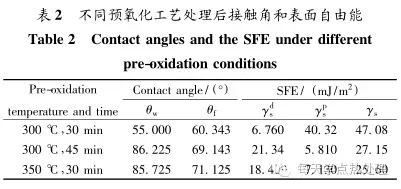
It can be seen from the table that the contact angle is the smallest when oxidized at 300 °C for 30 min, and the surface free energy is calculated by formulas (2) and (3). The higher the surface free energy and the more unstable the tissue, the more significant the infiltration effect in the subsequent nitriding process.
2. 5 Pre-oxidation infiltration model and mechanism discussion In the process flow shown in Figure 1, when the set pre-oxidation temperature is reached and air is introduced into the furnace, the surface of the sample is generated by sputtering and oxidation. More uniform distribution of oxide particles accompanied by microcracks and pore formation. The following reactions occur during this process:



in conclusion
1) Pre-oxidation has obvious permeation effect on ion nitriding, and the pre-oxidation process of optimal permeation effect is 300 °C, 30 min;
2) After pre-oxidation of the optimum process, ion nitriding at 500 °C for 4 h, the thickness of the compound layer reaches 15 μm, which is more than twice the thickness of the ion-nitriding compound layer without pre-oxidation and the same process; the thickness of the effective diffusion layer A maximum of 570 μm is achieved, which is significantly higher than the thickness of the effective diffusion layer of conventional ion nitriding;
3) After the optimal process pre-oxidation, a large number of uniformly distributed nano-scale oxide particles are formed on the surface of the material, accompanied by micro-cracks and holes, while the contact angle is the smallest and the surface free energy is the largest. These surface features facilitate the adsorption of nitrogen and further diffusion into the matrix, thereby effectively increasing the rate of ion nitriding;
4) The oxide formed by the optimal process pre-oxidation can be completely converted into nitride during the ion nitriding stage. Oxides formed by other pre-oxidation processes are difficult to completely convert into nitrides, and nitrides, oxides, and α-Fe phases coexist after ion nitriding.
Grinding Glass Microsphere
1. To select glass beads in types, sizes and quantity in accordance with viscosity, rigidity and dispersal of the grinded materials.
2. To clean glass beads and mill's inside before the grinding processed.
3. To input the grinded materials firstly and a curtain amount of glass beads later. To add continuously glass beads till 70%~80% of the mill is full.
4. To forbid to keep glass beads funning with little grinded material for a long time, as the glass beads inside the mill are easily broken at high speed operation.
5. To add new glass beads to ensure the quality and efficiency of grinded materials.
Grinding Glass Beads are mainly used for the disperser, grinding media of industries, such as coloring, paint, ink, coatings, resins, chemical engineering, with the advantages of smooth surface, even size, high hardness, good chemical stability. Thanks to the characteristics of heat-resistant, wearable, compression strength, the filling-type bead could be used to improve glass fibre reinforced plastic, rubber, and so on.
The glass bead can be produced based on the standard of countries or areas, such as EN1423/1424, AASHTO M247, BS6088, JIS R3301 and KS L2521 etc.


Grinding Glass Beads
Grinding Glass Beads,Grinding Glass Beads Borosilicate,Grinding Glass Beads Road Marking,Gj-I Grinding Glass Beads
CHIYE GLASS BEAD (HEBEI) CO., LTD , https://www.chiyeglassbeads.com
