Nanjing University's "seawater extraction of lithium" technology breakthrough
Recently, Professor He Ping and Professor Zhou Haoshen of Nanjing University published a research paper entitled "Lithium Metal Extraction from Seawater" online in the "Joule", a top academic journal in the energy field, on July 27, 2018. Driving energy, based on the combination of hybrid electrolyte (hybridelectorlyte) ideas and ion-selective solid thin film constant current electrolysis technology, successfully achieved the extraction of elemental lithium metal from seawater. The advent of this technology has opened up a whole new path for the development of marine lithium resources and the conversion and storage of too negative energy to chemical energy.
Lithium is one of the most important mineral resources in modern society, and is widely used in ceramic chemical industry, medicine, nuclear industry and the well-known lithium battery industry. With the popularity of electric vehicles and portable electronic devices, the size of the lithium battery market has grown significantly. It is expected that the next 30 years will consume 1/3 of the current global lithium reserves (Figure 1A), which will lead to the problem of insufficient supply of future lithium resources .
At present, the world's minable lithium reserves are all from ore and brine, totaling about 14 million tons. Refining lithium salts from ore and brine will consume a lot of energy and cause serious pollution problems. Compared to the limited lithium resources of ores and brines on land, 230 billion tons of lithium resources are stored in seawater, which is 16,000 times the total global lithium resources that can be mined (Figure 1B). Therefore, if simple, controllable and clean extraction of lithium from sea water is realized, humans will obtain almost inexhaustible lithium resources.
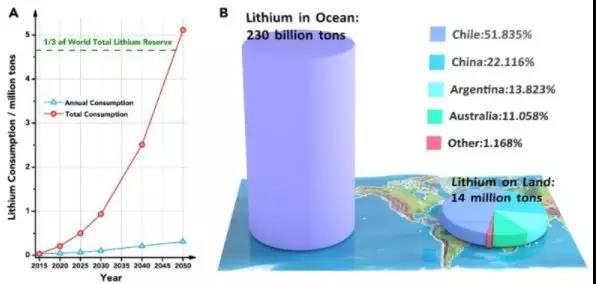
Figure 1: (A) Expected annual consumption and total consumption curves of lithium resources from 2015 to 2050; (B) Comparison of lithium resource reserves in oceans and land. The distribution of lithium resources on land is uneven, mainly distributed in Chile, China, Argentina and Australia.
Although seawater contains extremely rich lithium resources, the lithium concentration in seawater is very low, only 0.1 ~ 0.2ppm, which makes it difficult to extract lithium from seawater. Researchers have proposed many solutions, including adsorption and electrodialysis.
The adsorption method is to realize the adsorption of lithium from seawater through the exchange mechanism of some hydrogenated metal oxides with hydrogen ions and lithium ions. The electrodialysis method promotes the directional movement of positive and negative ions in seawater by applying an electric field, and then achieves the enrichment of lithium ions by selectively permeating the membrane.
Existing seawater lithium extraction technology has a slow extraction rate and is difficult to control. The initial extract obtained needs further processing to obtain metallic lithium or pure lithium compounds (such as Li2CO3). Therefore, the existing seawater lithium extraction technology may not be able to meet the large-scale demand for lithium resources in the future, such as lithium-sulfur batteries and lithium-air batteries.
Professor He Ping and Professor Zhou Haoshen of the School of Modern Engineering and Applied Science of Nanjing University proposed the concept of hybrid electrolyte (Hybridelectrolyte) as early as 2009. This concept combines the characteristics of organic and aqueous electrolytes and broadens the battery system compared to a single electrolyte Operating voltage and application range. Based on the combined electrolyte, the team developed new large-capacity batteries such as water-based lithium-air batteries, lithium-air fuel cells, lithium-copper batteries, and lithium flow batteries.
Recently, the research team applied the combined electrolyte strategy to the technology of extracting lithium metal from seawater. The combined electrolyte designed by the team consists of a combination of positive and negative regions. The positive electrode area is a lithium ion organic electrolyte protected by an argon atmosphere, with copper foil immersed in the electrolyte as the positive electrode; the negative electrode area uses seawater as the working electrolyte, and the Ru @ SuperP catalytic electrode as the negative electrode. A lithium ion solid electrolyte ceramic membrane is used as a lithium ion selective permeable membrane to separate the positive electrode area and the negative electrode area, and the ceramic film only allows lithium ions to pass through. A self-designed miniature tunable too-negative energy plate constant current power supply applies a constant current between the positive electrode and the negative electrode, so that the lithium ion source in the seawater of the negative electrode area continuously passes through the solid ceramic film to reduce the metal lithium on the surface of the positive electrode copper sheet , Successfully achieved the extraction of elemental lithium metal from seawater (Figure 2).
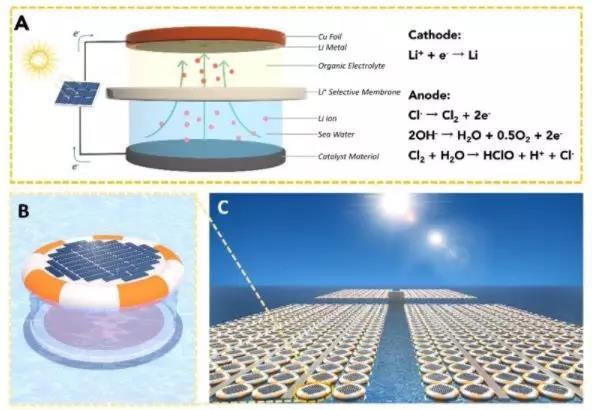
Figure 2: (A) Schematic diagram of the electro-hydraulic seawater lithium extraction device driven by too negative energy; (B) Schematic diagram of the device, from top to bottom are the too negative energy plate, the organic electrolyte positive electrode area, the ceramic selective membrane, In the negative electrode area of ​​seawater, the entire device can float on the sea surface with a rubber band; (C) An imaginary picture of the arrangement of a large number of devices on the sea.
During the electrolysis process, the reduction reaction of lithium ions occurs on the positive electrode:
Li ++ e- → Li
The oxidation reaction of seawater on the negative electrode:
2Cl- → Cl2 + 2e-
2OH- → H2O + 0.5O2 + 2e-
Cl2 + H2O → HClO + H ++ Cl-
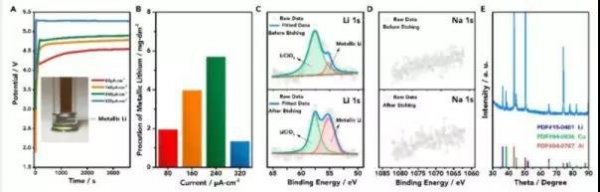
Figure 3: (A) Potential-time curves at 80, 160, 240, and 320 μA · cm-2 current density (the inset is a photograph of an electrode electrolyzed at 80 μA · cm-2 current density for 1 h); (B) per square Yield of lithium metal on a centimeter copper sheet; (C) XPS characterization diagram of Li before and after deposition of argon ion etching; (D) XPS characterization diagram of Li and Na of positive deposition product before and after argon ion etching; (E) deposition product XRD characterization diagram (Al peak comes from the sample stage of the atmosphere protection device)
In the process of extracting lithium from seawater, a silver-white substance is formed on the surface of the copper sheet. According to XPS and XRD analysis, the deposit on the surface of the copper sheet is metallic lithium. The electrolytic voltages at current densities of 80, 160, 240, and 320 μA · cm-2 are 4.52V, 4.75V, 4.88V, and 5.28V, respectively, and the production of lithium metal is 1.9, 3.9, 5.7, and 1.2 mg · dm-2 ·, respectively. h-1 (Figure 3).
When the current density exceeds a certain threshold, such as 320 μA · cm-2, a serious side reaction (decomposition of the electrolyte) occurs in the positive electrode, resulting in a decrease in lithium production. It can be seen that the technical advantage of the lithium extraction from seawater is that the lithium metal element can be directly obtained, and the chemical energy converted from too negative energy is already contained in the metal lithium element, which can be released through a new battery system such as a lithium-sulfur battery or a lithium-air battery.
In addition, the constant current electrolytic method is fast and tunable, and is suitable for large-scale production. The invention of this technology has opened up a whole new path for the development of marine lithium resources and the conversion and storage of too negative energy to chemical energy.
SUN YAT INDUSTRY LIMITED , https://www.ernte-eu.com
